NWE-Chance is assessing the feasibility for Heart Failure patients to get hospital-level care at home – average duration 5 days –with the support of a newly developed integrated technology platform.
All relevant stakeholders are involved in the project: hospitals, patients, MedTech companies and universities.
The project: first step
The project is developing an integrated platform to deliver home-hospitalisation to patients with acute decompensation of heart failure. Three MedTech companies work together to integrate their technology in an easy to use and safe platform for heart failure patients. The platform will be developed in such a way that other companies, developing new technologies, will be able to link and connect their innovations for home hospitalisation.
The project: second step
As second step, the project will validate the newly developed eHealth platform and assess the feasibility of the home hospitalisation strategy.
Jessa Hospital is taking the lead for this ‘feasibility study’ organised with three small scale pilots in three different hospitals in the NWE region.
During its first year of work, NWE CHANCE has developed the study protocol, defined the interventions and the assessment criteria for evaluating the feasibility of the home-hospitalisation strategy.
Stakeholders involved
The participating hospitals are ISALA Hospital in Zwolle (The Netherlands), Jessa Hospital in Hasselt (Belgium) and MUMC+ in Maastricht (The Netherlands). Hasselt University (Belgium) will scientifically support this study. During the three small scale pilots, 100 patients with an acute decompensation of heart failure will be included.
The intervention will be different for the three hospitals as a result of the level of experience with home hospitalisation.
ISALA Hospital has already ten years of experience with home-hospitalisation of heart failure patients. They have a well-structured organisation with specialised nurses who are trained to deliver home-hospitalisation treatment to patients. Therefore, ISALA will include 50 patients with acute decompensation of heart failure and will take medical decisions on the basis of the eHealth platform technology.
MUMC+ has no experience with delivering home-hospitalisation. However, they have already experience with providing infusion therapy at the home of the patients. MUMC+ will include 25 patients and will take medical decisions on the basis of the eHealth platform technology.
Jessa Hospital has no experience with either home-hospitalisation or remote infusion therapy. Jessa Hospital will include 25 patients at the end of their heart failure hospitalisation, and they will not provide infusion therapy at the home of the patient. So, in Jessa Hospital, we will study the feasibility of the home-hospitalisation organisational strategy for a hospital with no experience in home care.
The intervention
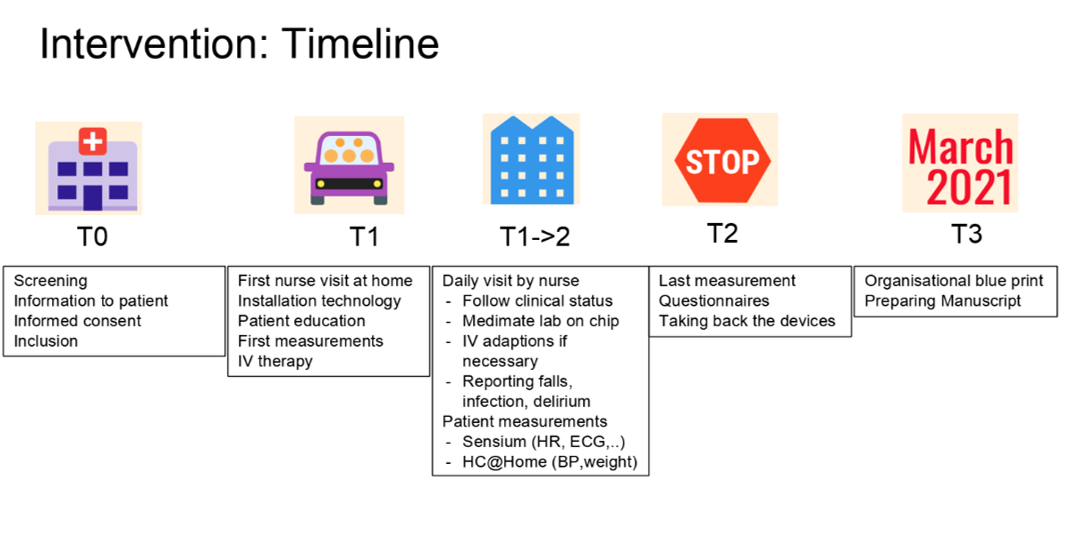
In general, the home-hospitalisation process will consist of 5 phases:
T0 for recruitment, T1 for the first home hospitalisation day, T1->2 for the home hospitalisation period, T2 for the end of the home hospitalisation and T3 for the long-term follow-up of 30 days to record all major cardiovascular events such as re-hospitalisation. During the home hospitalisation period, monitoring data will be collected from patch (heart rate, respiratory rate, posture, activity, …) and other devices (blood pressure and weight).
Caregivers can monitor these parameters on a clinical dashboard but also patients are able to monitor their parameters on a smartphone they receive during the home-hospitalisation.
The feasibility of this care pathway will be assessed with questionnaires on patients and healthcare professional's satisfaction, acceptation and usability.
An online research database, Castor, will be set up and electronic Case Report Forms will be prepared to collect all the study related outcomes of the three hospitals in one online database. UHasselt will perform a quality check on the data and will ensure that the timings and number of inclusions are reached by the end of the study.
The end of the study is planned in May 2021. On this moment, we are awaiting approval of the ethical committees of all participating centres.
